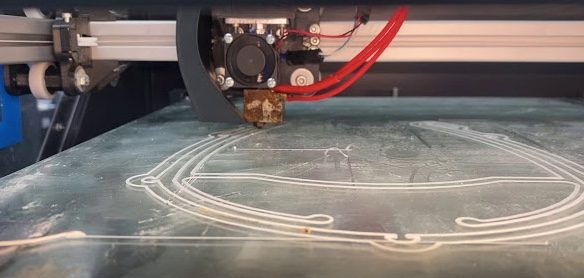
Collaboration in Action: How Georgia Tech faculty and the Flowers Invention Studio used GCMI and the Atlanta medtech ecosystem to bring desperately needed PPE to frontline healthcare workers.
In mid-March 2020, the shortage of personal protective equipment for healthcare providers responding to the COVID-19 outbreak had become a national emergency. By the time the University System of Georgia suspended on-campus instruction and asked students to depart campus by March 13 in response to rapidly increasing numbers of COVID-19 cases, Christopher Saldana, Ph.D., associate…