HUB HYGIENE
Successfully Guiding Medical Innovation
GCMI is proud to work in over 11 therapeutic areas and a wide array of medical products (Class I, II and III) including drugs and biologics. Below are some of the projects that we have had the pleasure to be a part of and further information on what roles we played in its development and to what result.
Please contact us today if you have any questions or are a physician innovator or engineer with an idea for a new device.

“CLABSI is an avoidable problem with significant unmet clinical need currently relying on ‘Civil War technology:’ disinfecting wipes coated with isopropyl alcohol,” Dr. Ready says.
Dr. Ready anticipates completion of the testing and formal submission of Hub Hygiene’s 510k regulatory submission to the FDA in this calendar year.
“GCMI does medical device development every day. They are agile, responsive and can often solve problems with minimal input given their expertise and experience. They absolutely accelerated our commercialization pathway.”
CHILDREN’S MERCY KANSAS CITY
Catheter Gripping Tool

No tool exists specifically for the purpose of removing line connections. Clinical staff ‘conveniently’ resort to using hemostats, gloves, towels or other random tools that act like pliers for the task. The unsolved problem has been, tubing expands and contracts with fluctuation in humidity and temperature causing connectors to bind. Breaking those connectors too frequently results in wasted time, unnecessary pain for the clinician and patient, and, in some cases, additional surgical procedures.
Armed with early-stage, yet detailed descriptions and renderings, 3D printed prototypes and very early test results produced in conjunction with Kansas State University, the Children’s Mercy Kansas City Innovation team engaged GCMI to evaluate the regulatory implications of the device on the development pathway.
“We anticipate full design history file creation including specifications, inputs, user needs and risk analysis in preparation for an FDA pre-submission meeting as the next step in our process,” John said. “We [further] anticipate the Children’s Mercy Kansas City clinical team will be the earliest adopters for user feedback and any functional design tweaks prior to manufacturing transfer and production scale up.
Read more about Children’s Mercy Kansas City’s journey with GCMI here.
MICRON BIOMEDICAL
Microarray Needle Technology
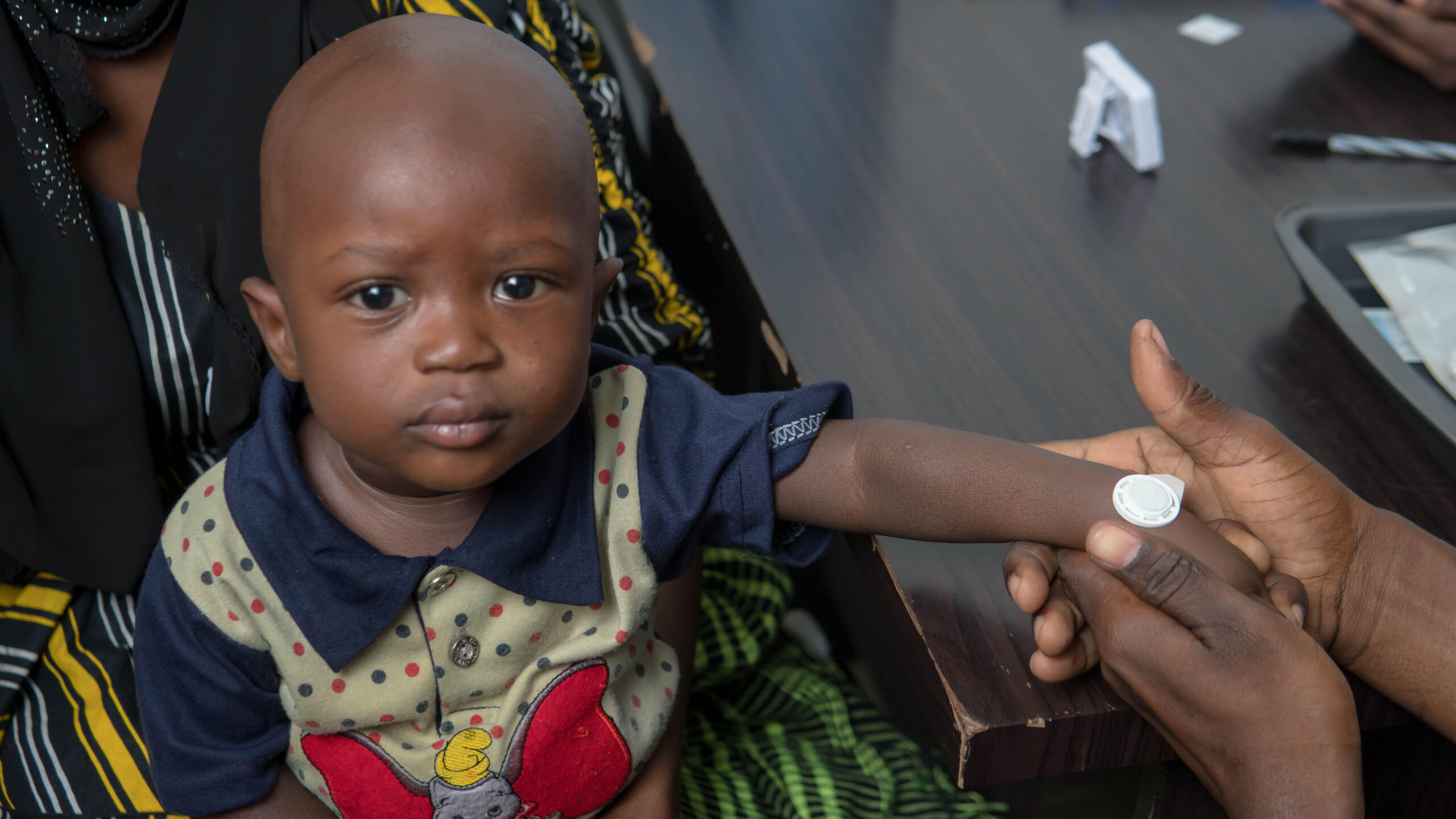
“Partners like BARDA, the CDC and the Gates Foundation charge us with mandates to solve problems with global impact. Be those national health security problems, pandemic, epidemic, or health issues rooted in problems of social access like measles and rubella, Micron leads the way to improving access. Having spent billions of dollars in response to the Covid-19 pandemic, BARDA now has equity investment in Micron via the Global Health Investment Corporation fund, a sign of how strongly they believe in the technology. Many of the leading minds in global health understand the magnitude of the impact our work has, and can, accomplish.
“This impact would not be possible without GCMI.”
JACKSON MEDICAL
GloShield™

SALDANA RESEARCH GROUP & GEORGIA TECH
PPE Face Shields

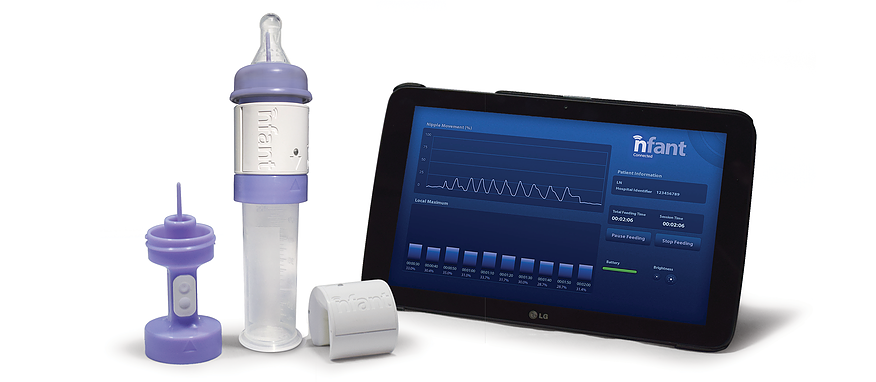
NFANT LABS
NeoNatal Feeding System
DR. SCOTT HOLLISTER, CHILDRENS HEALTHCARE OF ATLANTA
First 3D Printed Pediatric Tracheal Implant

EMORY CHILDREN’S PEDIATRIC RESEARCH CENTER / GEORGIA TECH BME
Re-expressing Pacemaking Cells for Cardiac Arrhythmia in Pediatric Patients

GEORGIA TECH BME FALL 2021 CAPSTONE: TEAM FIVE OF HEARTS
A Safer Way to Conduct Pericardiocentesis
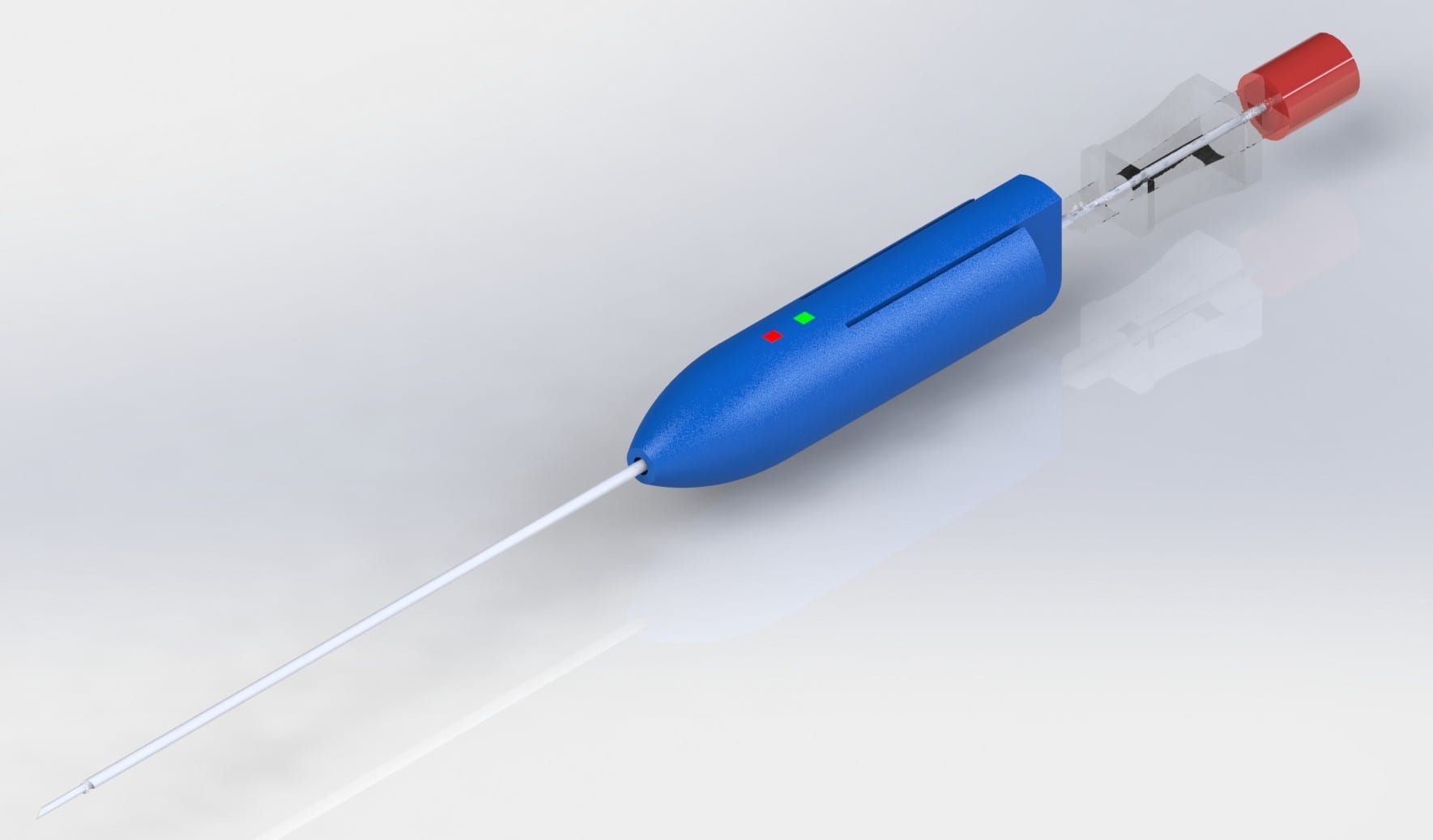

 GloShield is a reliable solution that reduces the risk of fires and burns in the operating room attributed to fiber-optic light cables. With seamless implementation onto existing cables, GloShield limits “never events” and prevents thermal injury.
GloShield is a reliable solution that reduces the risk of fires and burns in the operating room attributed to fiber-optic light cables. With seamless implementation onto existing cables, GloShield limits “never events” and prevents thermal injury.