With GCMI’s Help, Micron’s Microarray Needle Technology Nearer To Forever Changing the World for the Better
Medical device and biotech or medtech innovation doesn’t work like consumer electronics or software. It works more like aerospace. Because lives are literally at stake it needs more than a place for people to work, high speed internet, brilliant minds and dedication. It needs certified clean rooms, highly expensive equipment and advanced materials, intensive validation testing and rigor at every level of its pathway to commercialization and positive health outcomes.
What successful university bred biotech innovation looks like
Like many game-changing technological advancements, Micron Biomedical’s story begins in a public institution of higher education, its research labs and an unmet clinical need; this one global in scale. With origins in Professor Mark Prausnitz’ Laboratory for Drug Delivery at Georgia Tech, Micron Biomedical has taken a massive unmet clinical need – safe, effective, affordable delivery and administration of therapies and vaccines in every corner of the globe – and created a device technology proven in its potential to successfully meet that need.
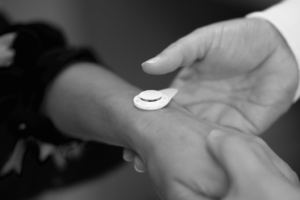 Put simply, Micron’s technology delivers traditionally injected vaccines and medicines without needles through a dissolvable microarray technology. It offers access to life-saving pharmaceuticals for children and adults and allows painless, self-administration of therapeutics and vaccines, in seconds to minutes—at home, in the field, and wherever they are needed—without the need for a medical professional to administer them, all with the push of a button. It eliminates or greatly reduces the need for cold chain during delivery and/or storage; reducing transportation and storage costs; allowing safe vaccine and drug administration by non-medical personnel; reducing medical waste; and offering needle-free solutions to address vaccine hesitancy and improve patient compliance.
Put simply, Micron’s technology delivers traditionally injected vaccines and medicines without needles through a dissolvable microarray technology. It offers access to life-saving pharmaceuticals for children and adults and allows painless, self-administration of therapeutics and vaccines, in seconds to minutes—at home, in the field, and wherever they are needed—without the need for a medical professional to administer them, all with the push of a button. It eliminates or greatly reduces the need for cold chain during delivery and/or storage; reducing transportation and storage costs; allowing safe vaccine and drug administration by non-medical personnel; reducing medical waste; and offering needle-free solutions to address vaccine hesitancy and improve patient compliance.
Since its inception in 2014, Micron has raised roughly $50 million dollars in non-dilutive funding, venture investment and R&D reimbursement from pharma and biotech collaborators for its demanding journey from the lab to patients. Funding includes seed stage support from the Georgia Research Alliance (GRA), a $23.6 million gift from the Bill and Melinda Gates Foundation in November 2023 along with a $17 million Series A round co-led by Global Health Investment Corporation (GHIC) and LTS Lohmann earlier the same year.
According to the company, “Micron partners with and/or receives funding from private and public pharmaceutical and biotech companies, the [aforementioned] Bill & Melinda Gates Foundation, the Centers for Disease Control and Prevention (CDC), the U.S. Center for Biomedical Advanced Research and Development Authority (BARDA), PATH and the Georgia Research Alliance (GRA).”
“Game changing potential in humanitarian settings”
In May 2024, The Lancet published positive Measles and Rubella (MR) Phase 1/2 trial data utilizing Micron’s novel vaccine delivery device. A comment in the same issue described the implications of the data and recognized microarray technology for its potential as “game-changing in humanitarian settings.”

Steven Damon, CEO, Micron Biomedical
“Thanks to the support and funding of our partners we are focused on ending preventable diseases in low and middle income nations,” Micron Technologies CEO Steven Damon told our colleague Paul Snyder. “Children are still dying of measles due to lack of access to vaccination. Vaccines need refrigeration in transit and they need qualified clinicians to administer them. Trial results with our microarray needle technology showed prevention against measles better than or equal to subcutaneous injections. These trials and results represent the highest levels of importance in global health improvement. Improved access to vaccinations that utilize Micron’s technology can save lives and may well eradicate measles worldwide.”
Micron’s microarray technology, combined with important vaccines and drugs, including measles and rubella, are manufactured at the Global Center for Medical Innovation (GCMI), a Georgia Tech affiliate.
“We are also focused on effectively addressing the next global pandemic with vaccines that can be administered in the home without the need for a healthcare professional instead of waiting in lines by the thousands at large gathering places,” Steven said.
“Partners like BARDA, the CDC and the Gates Foundation charge us with mandates to solve problems with global impact. Be those national health security problems, pandemic, epidemic, or health issues rooted in problems of social access like measles and rubella, Micron leads the way to improving access. Having spent billions of dollars in response to the Covid-19 pandemic, BARDA now has equity investment in Micron via the Global Health Investment Corporation fund, a sign of how strongly they believe in the technology. Many of the leading minds in global health understand the magnitude of the impact our work has, and can, accomplish.
“This impact would not be possible without GCMI.”
Micron has multiple clinical trials for important vaccines and drugs ongoing and planned with a focus on low and middle income countries as well as high income countries like the United States.
GCMI is the bridge on which startups traverse the cavern between research, development, commercialization and a healthier tomorrow.
Micron uses GCMI’s certified clean rooms for production of every single one of its microarray products. GCMI has also provided incubator space and engineering support services including prototyping and design for manufacturability input effectively since the company’s inception. Micron has hosted representatives from many major pharma/biotech, international and national foundations and government agencies at GCMI. Those leaders are now fully aware of GCMI’s affiliation with Georgia Tech and its value to medtech, pharma and biotech innovation.
Assets like GCMI bridge the gap for medical device researchers, innovators and startups for whom it is too early to invest in manufacturing facilities, but need products to generate data required by regulators to prove safety and efficacy, including for critical trials in humans.
 “Leveraging early stage, research and seed funding like the initial funds GRA awarded to Micron, we were able to further develop our technology and attract tens of millions of dollars in follow on investments, powerful evidence of the value and importance of GCMI to the medtech, pharma and biotech innovation ecosystem at local, regional, national and global levels,” said Steven.
“Leveraging early stage, research and seed funding like the initial funds GRA awarded to Micron, we were able to further develop our technology and attract tens of millions of dollars in follow on investments, powerful evidence of the value and importance of GCMI to the medtech, pharma and biotech innovation ecosystem at local, regional, national and global levels,” said Steven.
Access to GCMI creates value and leverages early stage funding that keeps researchers, innovators and startups advancing their technologies without having to seek larger investment from those unwilling to do so at an early stage. Increasingly, venture capital demands high levels of “de-risking” of new technologies. This creates a major disconnect between the funds required for de-risking activities (design, development and testing including products or test articles and facilities) and the data from the end points those activities generate as many investors demand. It’s a mountain that many promising new technologies fail to surmount.
If a young medical device company spends massive sums of investment dollars in manufacturing facilities only to have a pivotal or Phase III clinical trial fail, the game is over. There is no going back to solve the problem because the money’s gone and additional investment is highly unlikely if not impossible to acquire.
“We have kept the company alive and growing because we can actually provide enough product for the preclinical and human trials,” Steven said. “Every one of those clinical trial products comes from work we do at GCMI”.
“Without GCMI we could be years behind where we are now. We have also proven to our partners that we can deliver value and successfully meet our mandates, improving global health in a tangible way, changing the world for the better together.”
Forging ahead knowing this is what success looks like
Concurrent to the clinical trials in flight, Micron is also working on development of its own production facility, expected to come online in mid-late 2025. Until then, GCMI remains paramount to production of the company’s micronarray products eedle devices required to execute and complete upcoming trials.
 “For all parties concerned, Georgia Tech, the State of Georgia, our partners, the pharmaceutical industry and most of all patients and the unvaccinated in global locations for whom access has been an insurmountable challenge, this, this success is the desired result,” Steven said.
“For all parties concerned, Georgia Tech, the State of Georgia, our partners, the pharmaceutical industry and most of all patients and the unvaccinated in global locations for whom access has been an insurmountable challenge, this, this success is the desired result,” Steven said.
“Because of Mark Prausnitz, Devin McAllister, Sebastein Henry (Micron’s Founders), and his team, because of leaders at BARDA, the CDC, the Bill and Melinda Gates Foundation, the Georgia Research Alliance, GCMI and our team at Micron, we are on a path to provide access to important and life saving vaccines to tens of thousands of children in lower and middle income countries and millions more around the worldAnd in our next moment of global panic because of an illness that has not yet presented itself, we will meet the challenge with greater speed and saving of life than would otherwise have been possible.
“This is why we innovate. This is why we work together. This is why leaders invest in each other and foster innovation with high commercial potential. To make the world a better, healthier place. We are here and we’re nearer the goal thanks to GCMI’s resources and facilities.”
