Check out the work of Spring 2023 Capstone Team JAWWS and how GCMI supported their efforts to address a strong unmet clinical need.
 Few people, if any, actually enjoy being in the dentist’s chair, correct? If that’s true, certainly no one enjoys the oral surgeon’s operating room undergoing an open reduction and internal fixation (ORIF) procedure. And if that’s true, woe unto the person that has to undergo ORIF twice because of a hardware failure due to inaccurate mandibular bone thickness measurements.
Few people, if any, actually enjoy being in the dentist’s chair, correct? If that’s true, certainly no one enjoys the oral surgeon’s operating room undergoing an open reduction and internal fixation (ORIF) procedure. And if that’s true, woe unto the person that has to undergo ORIF twice because of a hardware failure due to inaccurate mandibular bone thickness measurements.
Because of a 9.5% re-operation rate due to postoperative complications, such as implant failure and infections, for approximately 21,000 ORIF procedures annually, that is indeed the case: an ORIF re-operation at the cost of $35,000 and weeks of additional recovery (and a strict soft food diet).
“One of the major obstacles to the procedure is selecting the appropriate screw length, ranging from 4 to 19 millimeters for each patient based on their unique bone anatomy,” states Yunha Ham, one member of the 2023 Georgia Tech Spring Capstone participant Team JAWWS.
The current measuring device standard is not well suited for the tight confines of the mandibular space, is prone to slipping in the surgeon’s hand and frequently results in abandonment of an accurate bone depth measurement in exchange for an educated guess for screw length selection. This is a major contributing factor to the ORIF procedure’s 11.4% complication rate and aforementioned 9.5% re-operation rate.
With support provided by GCMI, and the unmet clinical need presented by Dr. Joyce Xu and Dr. Shelly Abramowicz, Team JAWWS created “enGAUGE, a new surgical depth gauge featuring a double hooking mechanism that hooks on to the bone for more stable insertion and effective engagement that utilizes single hand operation.”
With three of the BME team members in the pre-dental breadth elective at Georgia Tech, and full team mutual interests in mechanical elements and strong potential for short-term clinical impact, selection of the project as presented by Drs. Xu and Abramowicz made perfect sense. But the team knew they would need additional testing and prototype production support to successfully verify and validate enGAUGE’s potential.
“We tested our earliest prototypes on wood, which made sense for the simplest, almost rudimentary needs,” said JAWWS team member Yunseo Ham. “But we knew we needed more anatomically appropriate materials and structures for testing in order to ultimately meet the clinical need, even for a relatively basic Class I regulatory pathway.”
[Editor’s note: Yes, indeed, Yunseo and Yunha are sisters.]
“We also needed access to prototype production resources including 3D and resin printers capable of handling the specification precision at a 1X scale instead of our 3X scale starting point,” said Ruiyang Zhao.
That’s where GCMI stepped in.
Preclinical Assets and Facilities Key to Meaningful Advancement in New Medical Technologies
“Testing on the porcine cadaveric structures taught us the soft tissues further restricted the use of the device to the exact drill bit dimension intended,” said Xinyu Chen. “But with some minor design modifications the device can have replaceable tips, which then as long as the tip chosen has matching dimension with the drill bit used by the physician, the device will operate efficiently. The prototype’s corresponding drill bit size (2mm) is the most common dimension used in mandibular fixations.”
“We also learned that maintaining a 3X size factor for the device’s body is advantageous for grasping and we can reintegrate that into the device’s design while maintaining 1X size factors for the measurement elements that must meet those specifications,” Yunha said. “This learning, along with the ability to compare our device side-by-side with the device currently on the market suffering from the unmet need we have addressed not only demonstrated ours’ advantages, it will almost certainly improve its utility and performance.”
“Our testing at T3 Labs also revealed additional time required for measurement taking,” Yunseo Ham said. “Surgical settings are unique and time feels quite condensed, especially compared to the bench. Because speed for these measurements is a strong user need, we increased the size of the numbers on the device and added a coloration element to improve visibility, accuracy and speed of measurement taking.”
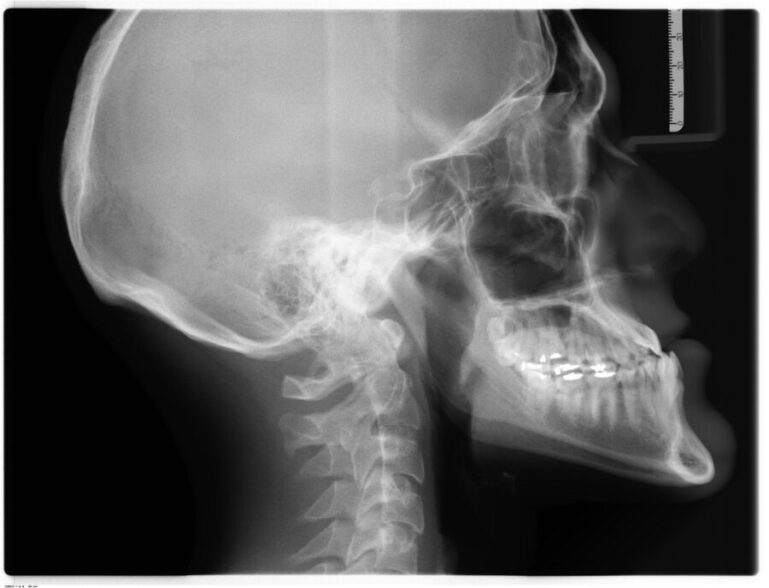
“X-rays supporting our testing at GCMI and T3 Labs clearly showed where the device engaged and the tip aligned at the bone level,” Ruiyang said. “This kind of evidence is important for our design history file, something that should make our regulatory journey and overall commercialization pathway more efficient.”
The team said next steps include consideration for measurement of fatigue and replacement requirements, especially if the device is to be sterilized for re-utilization. However, re-utilization would require documentation of ‘use cycles,’ an additional burden for the clinical team, which may make single-use indication a better solution to the unmet clinical need overall.
“Emory Hospital and the sponsors will hold the pending provisional patent, though we will all have our names on it,” Ruiyang said. “It will need additional design refinement, preclinical testing for accuracy and validation that it meets the user’s needs prior to adoption in the clinical setting, which is, of course, the goal.”
What’s next for Team JAWWS?
Ruiyang intends to enroll in the Dental College of Georgia this July. Xinyu will pursue a Masters in Biomedical Informatics at Harvard Medical School. Qingyu intends to pursue a Ph. D. in biophotonics at Johns Hopkins University. Yunha and her sister Yunseo will prepare for dental school.
The GCMI team thanks the team for sharing their Capstone journey with us here and wishes them all the best in their future endeavors.