A materials scientist and six GT students take aim at Civil War technology still ubiquitous in clinical care and its implication in 30,000 deaths every year.
The technology that has become Hub Hygiene’s easySCRUB started as a challenge issued to material scientist Jud Ready, PhD, in 2015 by a friend’s spouse, a North Carolina nurse.
Around that time, instances of Central Line Associated Blood Stream Infection (CLABSI), one significant subset of the highly problematic issue of hospital acquired infections (HAIs), or nosocomial infections, had finally become enough of a problem to draw the acute attention of clinicians, hospitals, healthcare administrators, and payers such as medicare or private insurers.
“Jud, can you help fix this CLABSI problem?” the spouse asked in a fateful email almost a decade ago.
From the Hub Hygiene team’s white paper:
A hospital acquired CLABSI occurrence typically increases the patient stay by 7-25 days, with a mortality rate of 10-40%. There are typically 250,000 – 500,000 CLABSI cases reported in the USA each year, with ~80,000 of those occurring in intensive care units (ICU), and almost always attributed to a lack of proper sanitization during central venous catheter (CVC) or peripherally inserted central catheter (PICC) maintenance. ICU patients are particularly susceptible to CLABSI, as these critically ill patients often require hundreds of central line accesses per day for repeated administration of vasoactives, antibiotics, blood products and blood draws.
CLABSI is categorized by insurance underwriters as a “never event” because it is a serious and largely preventable hospital acquired infection (HAI). Beyond the ~30,000 deaths/year attributed to CLABSI, medical costs associated with CLABSI treatment are not reimbursed by insurance and must be borne exclusively by the hospital, at an average cost of ~$45,000 per instance. Some estimates place this combined cost well above $10B annually for just ICU-based CLABSI treatments. Since each CLABSI occurrence is often attributed to negligence on the part of the caregiver, there are almost always accompanying litigation costs (~$9B/yr. combined nationally). Further financial penalties are borne by 25% of US hospital’s due to the Affordable Care Act, which penalizes hospitals with CLABSI rates in the bottom quartile of the nation by reducing their overall Medicare reimbursements for all procedures on all patients by 1%. This equates to an average loss of ~$500 per discharged patient3 from ~1,250 hospitals. With the bottom quartile of hospitals in the USA discharging approximately 8 million patients per year,4 this results in a financial loss of at least $4B/yr. If the unreimbursed direct costs of treatment, escalating litigation costs, and federal penalties are combined, CLABSI is at least a $24 BILLION financial problem for ~5,000 hospitals in the USA.
In the intensive care unit, Dr. Ready estimates an intravenous (IV) catheter access event occurs roughly every 15 minutes, or around 100 times per day per patient. Each access is an opportunity for hospital- or patient-borne pathogens to get into the IV. Beyond the ICU, IVs and their associated cleaning needs are prevalent in dialysis clinics, oncology wards, and anesthetics including maternity epidurals. Patient-conducted cleaning of permanently installed ports and connectors is also a strong area of concern with potential for infections arising in patients with permanently installed ports who regularly do their own cleaning and maintenance.
Civil War technology is still the standard of care. How is that possible?

Jud Ready
Current clinical best practices for sterilization of catheters, hubs and other connectors consists of “scrubbing the hub” for 15 seconds with a common isopropyl alcohol (IPA) soaked wipe. The problem is, numerous studies have found that the average time a clinician scrubs the hub is significantly lower than 15 seconds. Further, the non-conformal design and non-woven materials of those wipes is inadequate for thorough cleaning and removal of CLABSI causing bacteria in the nooks, crannies, and threads of the IV connectors. Instead of “smearing” contaminants around on the surface of a connector or hub, easySCRUB traps, lifts and removes the destroyed pathogens.
“CLABSI is an avoidable problem with significant unmet clinical need currently relying on ‘Civil War technology:’ disinfecting wipes coated with isopropyl alcohol,” Dr. Ready says.
Another GT Capstone project shows strong commercial promise and and spins out a startup
In 2016, Dr. Ready challenged a cohort of a half dozen students in Georgia Tech’s Capstone Design to create a solution for the CLABSI problem and its hundreds of thousands of occurrences, its tens of thousands of associated fatalities, and billions of dollars in avoidable cost to the U.S. healthcare system.
With GCMI’s help, including our colleague Mike Fisher, now professor of the practice for Georgia Tech, the six students led by Dr. Ready ultimately delivered the first designs and prototypes of easySCRUB, which “consists of an individually packaged, single-use, sugar cube-sized, open-cell micro abrasive melamine foam sponge that is saturated with isopropyl alcohol. It is used in a scrubbing/twisting motion to promote and maintain cleanliness and sterility of the IV catheter hubs, ports, Luer locks, septum and/or add-on device during connection, access, and maintenance events.”
A winning solution
According to Hub Hygiene, in a simulation of poor-adherence to the ‘scrub the hub’ protocol, after only four twists in three seconds of scrubbing, easySCRUB successfully removes several orders of magnitude more CLABSI-causing pathogen colony forming units from a port, compared to traditional isopropyl alcohol-saturated nonwoven prep pads. The easySCRUB device also facilitates infection-control compliance and monitoring by emanating an audible ‘squeak’ when used as indicated. This reassures the caregiver, patient and their loved ones of proper IV maintenance in real time.
The team won the Best Overall Award at the Georgia Tech Capstone Design Expo in April 2016. They then went on to present at several conferences including the Molecules for Minions conference in June 2016 and the iMatSci Innovation Showcase in November 2016. Hub Hygiene later won the 5th Annual Pediatric Device Innovation Symposium in September 2017.
With support from BASF, the manufacturer of the micro abrasive melamine foam and further funding associated with the aforementioned pediatric innovation symposium award, and Children’s National Hospital, HubHygiene forged on in its development and commercialization pathway.
In January 2019, Hub Hygiene was granted United States patent 10,166,085 for easySCRUB.
Now for the hard part: scaling up manufacturing to satisfy regulatory testing and sales
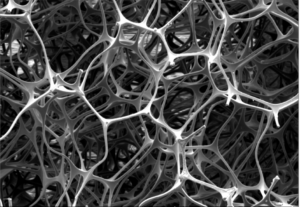
Hub Hygiene’s easySCRUB unique micro-structure.
“In our earliest stages, we bought substantially equivalent, though not sterilized, melamine foam, IPA, pouches, labels, and boxes from Amazon and ULine,” Dr. Ready told us. “Melamine is the same material used in dry erasers, which was useful for ‘show and tell’ and early prototyping, but nowhere near what we needed for clinical demonstrations or verification and validation testing.
“In 2023, we were effectively dead in the water with a substantial amount of additional FDA-required testing to go, but no samples to do it with since we had used our last gamma-sterilized samples in a test with BD.”
Hub Hygiene’s original sample lot used in those and other FDA-required tests was made by a contract manufacturer based in Minnesota. But thanks to Mike Fisher and GCMI’s expertise in requirements for testing, packaging, labeling and regulatory submissions, coupled with the help of the Center for Medtech Excellence and the Georgia Manufacturing Extension Partnership, the company has manufactured an additional production lot here in Georgia that is intended for the remaining tests required for formal FDA 510k submission, a requirement it must achieve prior to use in a hospital or other clinical setting involving human patient care.
“Without the help of these entities, including the quality systems, processes and access to clean rooms for production, the samples required for testing would have cost us at least $40,000 to manufacture and sterilize,” Dr. Ready said. ”Thanks especially to CMTE, that expenditure will be much, much lower.”
“And thanks to GaMEP guidance and support, our next round of funding through a convertible note will not only carry us through the completion of testing and the formal FDA 510k submission, it will take us into production of additional lots of easySCRUB’s that will be sold and delivered to the providers that need them in order to drastically reduce the occurrences of CLABSI and its high volumes of negative, avoidable downstream effects.”
Interested investors in the funding round are encouraged to contact Hub Hygiene CEO Dr. Jud Ready via the hubhygiene.com website.
Dr. Ready anticipates completion of the testing and formal submission of Hub Hygiene’s 510k regulatory submission to the FDA in this calendar year, pending successful funding of at least a portion of the convertible note.
What others can learn from a material scientist’s venture into medtech innovation
“Pay attention to your quality management systems early. It will make transfer to production endlessly easier if you do. It is possible to over-invest there too, though. A shovel can do the job of an excavator – especially in the early years.”
“If you’re not experienced in the field of medtech innovation to begin with, know that it has its own vocabulary and processes, especially in the regulatory sphere. I’m a material scientist, and did my PhD in electronics reliability. My research today at Georgia Tech involves solar cells going to the lunar south pole next year. The medical field was entirely new to me and required its own education. GCMI can be especially helpful for innovators new to medtech.”
“Anticipate at least double the cost and double the time required to reach each milestone. Keep as much powder dry as you can. That brings up another acumen you will need, fundraising and financial fluency. What’s a convertible note? Is it preferable to other types of equity or funding mechanisms? Have I really exhausted every other source of reasonably available non-dilutive funding? Those are questions you will have to answer if you successfully achieve the milestones that precede them.”
What else would you tell others about GCMI’s capabilities, when to reach out to them and why?
 “The earlier you reach out to GCMI for guidance, the easier your journey is going to be,” Dr. Ready says. “What will your device look like when it gets to the FDA? What’s needed for testing? What regulatory classification should it follow? What about labeling? What about manufacturing at a scale of many millions per year? You’re going to need to know this, and much more, sooner rather than later if you are to be successful. Your investors are going to demand answers and accountability to those questions as well.
“The earlier you reach out to GCMI for guidance, the easier your journey is going to be,” Dr. Ready says. “What will your device look like when it gets to the FDA? What’s needed for testing? What regulatory classification should it follow? What about labeling? What about manufacturing at a scale of many millions per year? You’re going to need to know this, and much more, sooner rather than later if you are to be successful. Your investors are going to demand answers and accountability to those questions as well.
“The quicker you ask and answer the most relevant, pressing, potentially costly questions, the more cost and time efficient your work will be, not to mention preventing headaches and heartache down the road. GCMI does medical device development every day. They are agile, responsive and can often solve problems with minimal input given their expertise and experience. They absolutely accelerated our commercialization pathway.”
Get in touch
GCMI is fully committed to our customers’ success and welcomes you to contact us at any point in a technology’s pathway from the ‘back of the napkin’ to the bench, manufacturing, bedside and beyond. Whether you’re an individual innovator, startup or health system with an internal innovation program, initiative or ecosystem, get in touch. It’s never too early.
