GCMI’s Facilities Director Hugh Lambert has spent 11 years ensuring the efficiency of building operations and equipment, maintenance, and facilities management. He uses his 40+ years of experience along with his team to keep our industry leading 31,000 square foot preclinical facility performing at the highest of standards for our medtech and life science customers.
There are many demands to meet the various regulatory requirements of a facility like this. There is a balance of compliance while maintaining leading practices, industry benchmarks and trends. For instance, maintaining AAALAC accreditation. For instance, there are costs to control including power for HVAC systems, water consumption, daily cleanliness and customer experience requirements, OR equipment maintenance and certification, shipping, receiving, storage, IT, audio visual (AV) and on it goes.
Let it flow
One of the many deliverables of compliance for the facilities team at GCMI is AAALAC ’s standards for recycled air and air circulation state, “The use of 100% non-recycled air in the Vivarium is required for ventilation for animal holding areas and Surgical areas.” All air supply is treated with UV light and HEPA filters are used on all surgical air supplies. Only 25% recycled air is allowed in Administrative areas and it also receives UV light treatment.
In a hospital environment, every $1 spent on energy represents $20 spent to support systems and infrastructure, Hugh says. This same philosophy applies to T3 Labs, the CRO of GCMI. “We keep a very close eye on air turns,” Hugh says. “Our lab HVAC systems including all controls provided 100% approval from AAALAC in 2019. Our HVAC control practices makes the efficient, reliable functioning of these systems all the more important to our customers, approved visitors and staff.”
After a complete system upgrade GCMI is now taking full advantage of 40 to 50 percent reduction in HVAC associated costs totaling an estimated $1.5 million over a seven year period. The $1.5 million dollar savings is important but greatly magnified if you relate it to the $20 dollars saved for money not spent on support systems and infrastructure ($1.5 million x $20.00 = $30 million in sustainability. Earning GCMI a “Top Performer for Energy Conservation” by the Atlanta Better Building Challenge for significant utility reduction of 41 percent. Through a process of recalibration and system rebalance the facility has also reduced water consumption by nearly 70 percent since 2010
Certified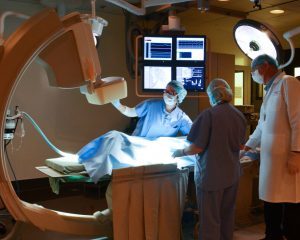
Ensuring equipment is working at their best level is key to ensuring the success of a program. The facilities team is responsible for maintaining the service and documentation for all equipment in the facility. Hugh says. “Through in-house experts or third party service providers, we maintain those certifications religiously. When we have evaluations or audits, we know everything we need is current, ready and in its right place.”
Hiding in plain sight
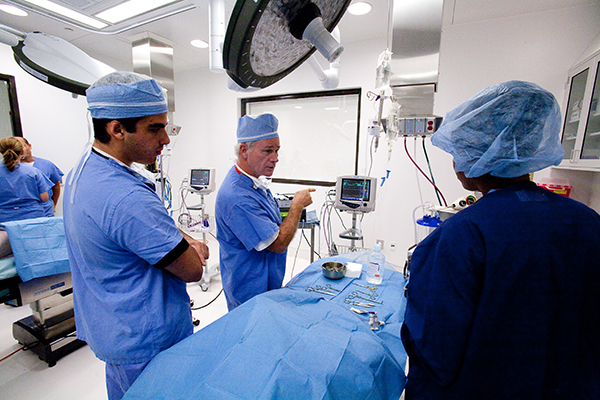
There are so many things that happen behind the scenes to ensure a facility like this runs smoothly. From daily cleanliness and comfort requirements, OR equipment maintenance and certification, shipping, receiving, storage, IT to audio visual (AV).
“Kind of like an umpire or referee, if those functions appear invisible, it means we’re doing our job well. But industry leading preclinical facilities take more than most know to operate at AAALAC standards and in a way that keeps our customers and their physicians coming back time and time again.”
If you are interested in learning more about our industry leading preclinical CRO services and facility, email info@gcmiatl.org.